 |
| John Dalton |
English chemist and physicist Dalton was a huge a neighborhood of figuring it all out. On this date in 1803 he wrote some notes on “ultimate particles” (also mentioned as “atoms,” which aren't all that ultimate, after all, since we now know that atoms are made up of smaller particles). Dalton proposed the thought that there are fundamental kinds of stuff, called elements, which each element is made from atoms of varied masses. Atoms of gold, as an example , would be much heavier than atoms of hydrogen. As Dalton wrote a couple of kind of elements, he used symbols to represent each element.
And that's the first time that anyone used a logo to represent an element!
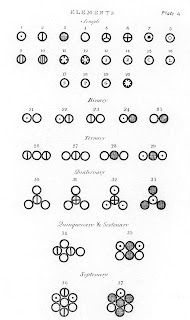
We don't use Dalton's symbols anymore, but many of his symbols were initial letters surrounded by circles, like S for silver, C for copper, and Z for zinc. We do use this general idea: nowadays, one or two letters (without the circle) is that the symbol for each element.
Our modern symbology was created by Swiss scientist Jons Kakob Berzelius, and naturally he didn't use English because the idea for his symbols, as Dalton did. Instead, he used the shared language of science of his time, Latin, because the idea for his symbols. thanks to this Latin origin, variety of our modern elemental symbols don't match up with English names:
Iron = Fe (ferrum)
Silver = Ag (argentum)
Gold = Au (aurum)
Lead = Pb (plumbum)
Potassium = K (kalium)
Copper = Cu (cuprum)
Antimony = Sb (stibium)
Mercury = Hg (hydrargyrum)
Tungsten = W (wolfram)
Tin = Sn (stannum)
Sodium = Na (natrium)
History:-
 |
| Dalton Introduces Atomic Symbols |
1803:
English chemist-physicist Dalton starts using symbols to represent the atoms of varied elements.Dalton, considered the daddy of recent atomic theory, made a logbook entry that day titled, "Observations on the last word Particles of Bodies and their Combinations." it had been the first use of symbols to represent the weather of recent chemistry.
He soon had a table of 21 elements arranged by mass , which he presented during a scientific paper the next month. Eventually, he had 36 different symbols.
 |
| Atomic Symbols |
In his 1805 work, "A New System of Chemical Philosophy," Dalton propounded the tenets of his atomic theory:
The chemical elements are made up of atoms.
The atoms of a component are identical in mass.
 |
| Helium atomic structure |
Atoms of varied elements have different masses.
Atoms combine only in small, whole-number ratios like 1:1, 1:2, 2:3, etc.
Atoms cannot be created or destroyed.
 |
| Atomic Symbols |
Dalton's symbols weren't those we use today, but circles containing distinct symbols (a dot for hydrogen, a cross for sulfur), or circles containing letters (C for copper, L for lead). He used them singly to represent elements and together to means compounds.
A decade after Dalton formulated his symbols, Swedish chemist Jöns Jakob Berzelius simplified the system. half Dalton's symbols used letters inside a circle to represent the element. Berzelius organized 47 elements with letters alone, and he based those letters not totally on English names, but on the Latin ones. In an era when all Europe's learned men (and the few women who were allowed into schools and universities) knew Latin, the shared language was a world interlanguage .
 |
| Dalton's Signature |
All but a few of Berzelius' symbols are still used today. So it's Au for gold and Ag for silver, not the circled G and S of Dalton's original notation.
The simplified notation led the way for English analytical chemist John Newlands to formulate his Law of Octaves and a prototype table of the weather in 1864, but it had been Russian chemist Mendeleyev who really laid it all on the table with 63 elements in 1869. When he flipped his chart to a horizontal table two years later, he created a form very almost like what you see in chemistry textbooks and on the walls of chem labs today.
Alas, Mendeleev's table was supported mass rather than number , so details a bit like the location of tellurium and iodine didn't compute . He thought it had been a problem of inaccurate measurement or other experimental error. it had been 1913 before English physicist Henry Moseley reorganized the table by number .
As for Dalton, his name lives on as alternate designation for the unit of measurement or amu. Microbiologists and biochemists need a convenient measure for large organic molecules. Kilo-u or kilo-amu would be awkward, so a molecule might be said to possess a mass of 35 kilodaltons, or kDA.
But it's Berzelius' symbols and what they mean that plague first-year chem students: you've to "get it" before you'll do anything .
Dalton's Scientific Work:-
Meteorology EditDalton's youth was influenced by a prominent Quaker, Elihu Robinson, a competent meteorologist and instrument maker, from Eaglesfield, Cumbria, who interested him in problems of mathematics and meteorology. During his years in Kendal, Dalton contributed solutions to problems and answered questions on various subjects within the Ladies' Diary and thus the Gentleman's Diary. In 1787 at age 21 he began his meteorological diary during which , during the succeeding 57 years, he entered quite 200,000 observations. He rediscovered George Hadley's theory of atmospheric circulation (now mentioned because the Hadley cell) around now . In 1793 Dalton's first publication, Meteorological Observations and Essays, contained the seeds of several of his later discoveries but despite the originality of his treatment, little attention was paid to them by other scholars. A second work by Dalton, Elements of English Grammar (or a replacement system of grammatical instruction: for the use of colleges and academies), was published in 1801.
Measuring mountains Edit:-
After leaving the Lake District , Dalton returned annually to spend his holidays studying meteorology, something which involved plenty of hill-walking. Until the arrival of aeroplanes and weather balloons, the only because of make measurements of temperature and humidity at altitude was to climb a mountain. Dalton estimated the height employing a barometer. The Ordnance Survey didn't publish maps for the Lake District until the 1860s. Before then, Dalton was one of the few authorities on the heights of the region's mountains.[8] He was often amid Jonathan Otley, who also made a study of the heights of the local peaks, using Dalton's figures as a comparison to ascertain his work. Otley published his information in his map of 1818. Otley became both an assistant and a devotee to Dalton.Colour blindness Edit:-
In 1794, shortly after his arrival in Manchester, Dalton was elected a member of the Manchester Literary and Philosophical Society, the "Lit & Phil", and a few of weeks later he communicated his first paper on "Extraordinary facts concerning the vision of colours", during which he postulated that shortage in colour perception was caused by discoloration of the liquid medium of the eyeball. As both he and his brother were colour blind, he recognised that the condition must be hereditary.Although Dalton's theory lost credence in his lifetime, the thorough and methodical nature of his research into his visual problem was so broadly recognised that Daltonism became a typical term for colour blindness . Examination of his preserved eyeball in 1995 demonstrated that Dalton had a less common quite colour blindness , deuteroanopia, during which medium wavelength sensitive cones are missing (rather than functioning with a mutated kind of pigment, as within the most typical kind of colour blindness , deuteroanomaly). Besides the blue and purple of the optical spectrum he was only able to recognise one colour, yellow, or, as he said during a paper.









0 Comments